Gmp good manufacturing practices for food manufacturers good manufacturing practices always going to be a hot topic.
Good manufacturing practices food processing ppt.
Good manufacturing practices and training.
Goodgood manufacturinmanufacturin g practicesg practices biltehqeeqgood management practices dec 07 11 good manufacturinggood manufacturing practices gmps practices gmps you can control many of the potential hazards in ayou can control many of the potential hazards in a processing plant by using aprocessing plant by using a standard set ofstandard set of principles and hygienic practicesprinciples and hygienic practices for thefor the manufacturing and handling of food.
It is designed to minimise the risks involved in any food cosmetic or pharmaceutical production that cannot be eliminated through testing the final product.
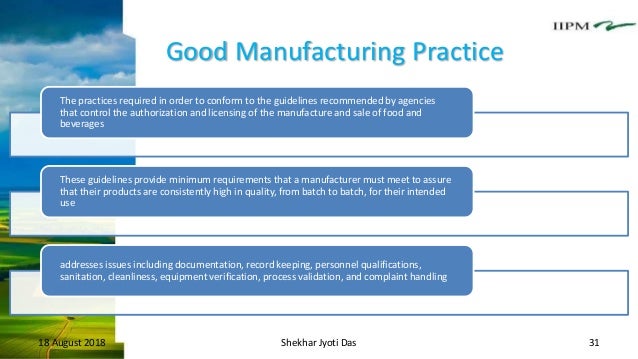
Introduction gmp ood anufacturing ractice it aimed to ensure that quality and safety are maintained throughout a process and thus prevent product rejection and financial losses.
It is fundamental for the construction of a food safety system in the manufacturing process.
Every food manufacturer needs to comply with this basic regulation.
Fig 4 gmp handbooks for every industry 5 good manufacturing practices worldwide enforcement.
In 21 cfr part 117 fda established a cgmp regulation as part of the current good manufacturing practice hazard analysis and risk based preventive controls for human food rule.
Good manufacturing practices in food processing 11 23 20161 gmp s in food processing 2.
Good manufacturing practice is a set of regulations codes and guidelines for the manufacture of drug substances and drug products medical devices in vivo and in vitro diagnostic products and foods.
The current good manufacturing practices cgmp coalition was founded in 2004 when it began working with the food and drug administration fda to move forward with revising the existing current good manufacturing practices cgmps 21 cfr 110.
Good manufacturing practices in food processing 1.
Good manufacturing practices gmps describe the conditions and practices that are necessary for the manufacturing processing packing or storage of food to ensure its safety and wholesomeness.
Good manufacturing practices for buildings facilities equipment production and process controls for foods 21 cfr 110 20 to 110 93 packers should also follow safety standards outlined in fda s food code ref 4 operator outside us should follow the same standards regulations and laws.
Good manufacturing practice good manufacturing practice gmp is a system for ensuring that products are consistently produced and controlled according to quality standards.
The current gmps comprise the basis for determining whether the practices conditions and controls used to process handle or store food products are safe and whether the conditions in the facility are sanitary.
Good manufacturing practices are enforced in the.