To ensure this the european union eu requires manufacturers of food contact materials fcm to comply with good manufacturing practices gmp.
Good manufacturing practices food europe.
The size of the business to prevent them being an excessive burden.
Ensuring safe edible food reaches the consumer is a complex undertaking.
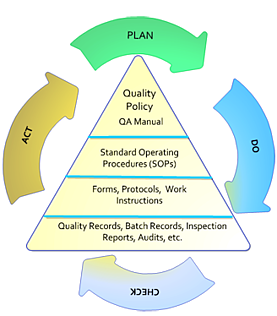
Good manufacturing practices gmps are the set of production standards that have been embraced by regulators retailers and consumers in the food and drug industries.
These practices are the fundamental operational requirements necessary to enable a food business to produce safe food.
Gmps provide a basic assurance that a product was produced under industry standard conditions.
Good manufacturing practice covers objects such as containers packaging paper cardboard ink and adhesives which could come into contact with food.
The staff s knowledge and skills and the organisation of the premises and equipment.
The retailer must be assured the complete supply chain for both foodstuff and packaging is compliant with necessary regulations and delivers a safe quality product to the end consumer.
Food gmps are the industry specific good manufacturing practices for food products.
Compliance with good manufacturing practice.
The authorisation holder must comply with the principles and guidelines of good manufacturing practice and use active substances active pharmaceutical ingredients which were manufactured in compliance with gmp.
Good manufacturing practice for manufacturers of food supplements the aim of this document is to produce guidelines which address the specific needs of the food supplement industry in relation to good manufacturing practice with special attention paid to the requirements of eu food legislation.
Eudralex volume 4 good manufacturing practice gmp guidelines volume 4 of the rules governing medicinal products in the european union contains guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use laid down in commission directives 91 356 eec as amended by directive 2003 94 ec and 91 412 eec respectively.
Good manufacturing practices gmp the manufacture or import of medicinal products is subject to manufacturing or import authorisation.